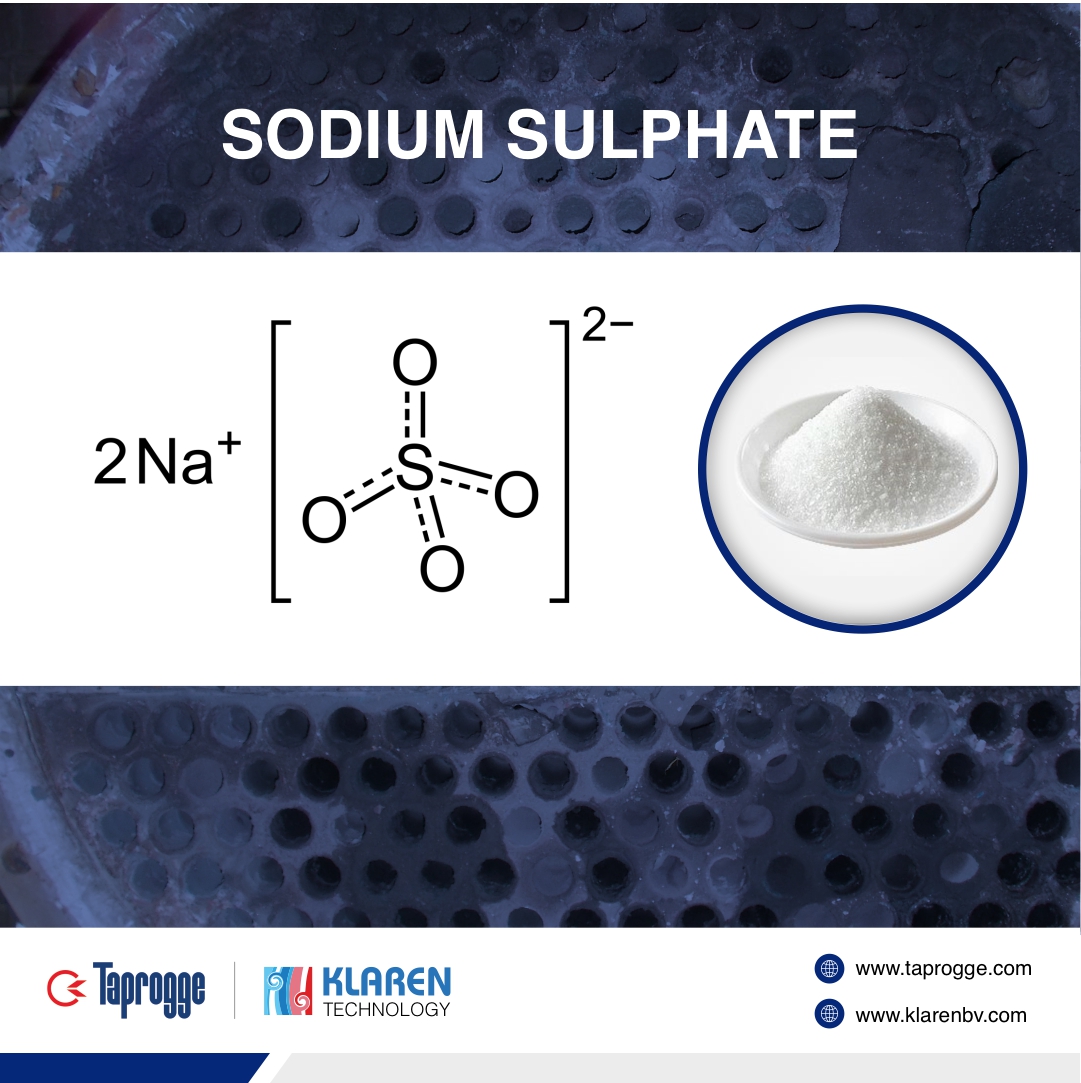
Sodium sulphate (also known as sodium sulfate or sulfate of soda) is an inorganic compound with the formula Na2SO4 as well as several related hydrates. All forms are white solids that are (highly) soluble in water, glycerol, and hydrogen iodide and insoluble in Ethanol.
Among all, the following are the major applications of this compound which are sold with minimum 99% purity:
- Use as filler material in Laundry Detergents;
- Production of Wood Pulp;
- Manufacturing of Glass;
- Coloring of Textiles.
Around 70% of sodium sulphate is produced from a natural source (Mirabilite) and the rest is a by-product of the production of chemicals.
This article discusses sodium sulphate production methods other than natural sources, the fouling problem and probable proven solution.
Production methods: Chemical processes
-
The most important chemical sodium sulphate production is during hydrochloric acid production, either from sodium chloride (salt) and sulfuric acid, in the Mannheim process, or from sulfur dioxide in the Hargreaves process. The resulting sodium sulphate from these processes is known as salt cake.
Mannheim: 2 NaCl + H2SO4 → 2 HCl + Na2SO4
Hargreaves: 4 NaCl + 2 SO2 + O2 + 2 H2O → 4 HCl + 2 Na2SO4
-
The second major production of sodium sulphate are the processes where surplus sulfuric acid is neutralized by sodium hydroxide, as applied on a large scale in the production of rayon. This method is also a regularly applied and convenient laboratory preparation.
2 NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2 H2O(l)
Byproducts:
-
Formerly, sodium sulphate was also a by-product of sodium dichromate manufacture, where sulfuric acid is added to sodium chromate solution forming sodium dichromate, or subsequently chromic acid.
-
Alternatively, sodium sulphate is or was formed in the production of lithium carbonate, chelating agents, resorcinol, ascorbic acid, silica pigments, nitric acid, and phenol.
Evaporation steps and fouling problem
The production of the anhydrous sodium sulphate often consists of an evaporation step where the concentration is increased above saturation limits. Once above saturation and in the presence of nuclei crystallization will occur. The crystals are subsequently removed from the saturated liquid using a separation step like for example by a centrifuge. Since the crystals removed will still be relatively wet, they need to be dried before they can be packaged.
In the process as described above the evaporation can be done in multiple arrangements of which a Multi Effect Evaporator (MEE) plant is one example.
The KLAREN self-cleaning heat exchanger technology is already applied in a MEE plant of a fertilizer producer (Asia) where sodium sulphate is recovered from the mother liquor as a by-product. In this MEE, fouling is the most dominant for effects 1 and 2, where the shell temperature is the highest. On the other hand, fouling is also observed in the last effect, but here it has a comparatively different nature. The scale formed is soft and fluffy, but it does have the tendency to clog complete tubes. The KLAREN system has been applied and has successfully eliminated fouling in the 1st effect.
We have learned from experience that when concentrations in an evaporator are pushed up to the formation of crystals along with the presence of impurities, fouling of the heat exchanger will occur and in these cases the KLAREN Technology has a strategic benefit over other available systems in the market.
Contact us today to learn more about the self-cleaning evaporator and how to achieve up to zero-fouling operations in your plant!

